Abstract
Introduction:
Although pivotal "driver mutations" have been described in T-cell lymphomas (T-NHL), targetable dependences are lacking for many entities. As result, T-NHL patients experience dismal therapeutic responses and have a poor survival. Therapeutic improvements have been hindered by the lack of informative models. Patient-Derived-Tumor-Xenografts (PDTX), recapitulating many of the biological features of primary cancers, represent a novel and powerful platform. Here, we constructed a library of T-NHL PDTX to foster drug discovery programs and new patient-tailored approaches.
Methods:
Fresh and/or viable cryopreserved samples from primary T-NHL were obtained from Turin, WCM, and MSKCC hospitals. For the initial tumorgraft implants, lymphoma fragments, PBMC or bone marrow cells were implanted in NSG-B2m mice. Seeds from arising tumors were then serially transplanted. MRI was used to follow tumor growth and dissemination. The lymphoma phenotype was assessed by multi-channel flow cytometry and immunohistochemistry. We used TCR gene rearrangement analyses, whole exome sequencing (WES), total RNAseq, and CHIP-Seq (STAT3, methylation marks) to annotate primary and PDTX samples. For the in vitro studies, PDTX were digested and stromal cells were excluded by panning. Enriched Patient-Derived Tumor Cells (PDTC) were cultured in either RPMI/20% FBS or StemSpan/10% KSR media. High-throughput drug screening was performed using a library of 432 compounds (1 µM for 72hrs). Cell titer glo/blue, trypan blue cell count, and annexin-V/7AAD were used to assess cell viability, apoptosis, and proliferation.
Results:
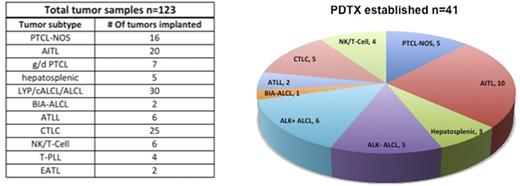
We implanted 123 fresh T-NHL samples, resulting in the generation of 41 PDTX lines (Fig.1). In addition, 14 implants led to the growth of lymphoblastoid EBV+ populations. All PDTX lines were successfully propagated (5-26 serial passages) with the exception of angioimmunoblastic T-cell lymphoma (AITL) PDTX, which displayed a delay in engraftment over time, and eventually ceased growing (passage 3-7). Serially propagated PDTX showed distinct immunophenotypic profiles, which closely reflected their corresponding primary samples. TCR gene rearrangement studies confirmed the faithful correspondence between donor and PDTX over different generations. WES and total RNA-Seq on primary and serially propagated PDTX (at least T1-T5) yielded a rich landscape of mutations shared by primary tumors and PDTX derivatives. On going mutations were also defined along propagations. CHIP-Seq assay defined a STAT3 classifier in STAT+ PDTC.
In vitro, all PDTC were shortly maintained (<7days), with some surviving for 6-8 weeks. To overcome their limited growth in vitro, we tested whether stromal cells (i.e. E4-Endothelial cells, E4-EC) could enhance their survival and/or growth. Indeed, co-culturing of PDTC with E4-ECs rescued cell death induced by ex vivo long-term propagation (peripheral T-cell lymphoma not otherwise specified and NK/T-Cell Lymphoma, PTCL-NOS and NK) or starvation (anaplastic large cell lymphoma, ALCL).
PDTC were also used to perform a drug screening targeting major signaling pathways (>3x replicates of 8 ALCL, 1 NK, and 2 PTCL-NOS), demonstrating that each PDTX exhibited a unique drug sensitivity profile, mostly dictated by its genomic landscape. This was epitomized by two PDTC (IL-2 and IL-19) derived from the same PTCL-NOS patient bearing an activating V658F JAK1 somatic mutation. These two PDTC, once exposed to JAK inhibitors, displayed down-regulation of pSTAT3 (24hr) and massive cell death (T3-T6, >95% at 72hrs). Similar results were seen with ALK inhibitors in ALK+ ALCL PDTC (n=5). Moreover, specific drugs were identified to bypass refractory phenotypes in selective PDTC (i.e. proteasome and topoisomerase inhibitors).
Conclusions:
We built up a large and representative PDTX library from major T-NHL entities. Immunophenotyping, genomics and functional studies showed a close correspondence between primary samples and correspondent PDTX derivatives. Our in vitro models provide a starting point for elucidating stromal:tumor cell interactions that contribute to lymphoma growth and survival in vivo, and evidence that a drug screening can be successfully executed on PDTCs. We predict that this cohort may be a valuable tool for testing novel therapeutic compounds and fostering personalized therapeutic approaches in T-NHL patients.
Thompson: Agios Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Other: Founder; Merck: Membership on an entity's Board of Directors or advisory committees; Charles River Laboratories: Membership on an entity's Board of Directors or advisory committees. Aster: Eli Lilly and Company: Membership on an entity's Board of Directors or advisory committees. Ruan: Cell Medica: Research Funding; Pharmacyclics: Research Funding; Seattle Genetics: Consultancy, Research Funding; Celgene: Consultancy, Research Funding. Dogan: Seattle Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Peer Review Institute: Consultancy; Celgene: Consultancy; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche Pharmaceuticals: Consultancy. Cerchietti: Celgene: Research Funding; Lymphoma Research Foundation: Research Funding; Leukemia and Lymphoma Society: Research Funding; Weill Cornell Medicine - New York Presbyterian Hospital: Employment. Horwitz: Celgene: Consultancy, Research Funding; Kyowa-Hakka-Kirin: Consultancy, Research Funding; Infinity/Verastem: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; BMS: Consultancy; HUYA: Consultancy; Aileron Therapeutics: Research Funding; ADCT Therapeutics: Research Funding; Millenium/Takeda: Consultancy, Research Funding; Mundipharma: Consultancy; Forty-Seven: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal